
Even if you add in that respiration is exothermic and there is exothermic transmutation, there is a missing input.
A 150 pound man needs at least 17,000 Calories, proportionally more if heavier, merely to be a warm blooded man.
Where does this energy and the rest required for life come from? Is there only one source?
The missing input obviously is not visible to our senses; but, yet it exists or we would not.
The missing input is frequency energy, effectively from God and His Creation. God's Love (life force) constantly
supplies us with something to maintain our lives.
We as man while respirating (re-spiriting with each breath of God) are inseparable from God's Love (life force).
God made us self healing. If you get a cut, it does not last a lifetime.
Solar and cosmic radiation on a microscopic scale cause the DNA cell nucleus to vibrate in an electrically conductive
salt solution encased in a cell wall membrane that is an electric insulator that has no where to go ergo it reduces to
heat within the cell. Life is vibration. Dead cells by definition do not vibrate. The Secret of Life, Georges Lakhovsky
Traditional Chinese Medicine integrates Mind, Body and Spirit. Mind and Body are not enough. We need to address the
Spiritual and Etheric as well.
DNA is a biocosmic resonator which sends and receives, i.e. prayer is a direct communication and most likely in direct
resonance with God. Dr. Lenoard Horowitz
There are trillions of cells that make up your body. For the moment I want you to think about just one. That one cell
is incredibly busy. In just the last second there were over 100,000 chemical reactions that occurred in this cell. Now,
step back and consider your body as a whole. The sheer volume of activity happening inside you at any given moment is
almost incomprehensible. With so much information being processed all at once, it's fair to ask how it all works.
Modern biochemistry now admits that a single cell is more complex than New York City being able to duplicate itself
and create thousands of chemicals.
If you want to find the secrets of the universe, think in terms of energy, frequency and vibration. Nikola Tesla
Future medicine will be the medicine of frequencies. Albert Einstein
In the end the answer will always be pretty simple. Albert Einstein
Of course God sustains us. Do we know all of the ways God sustains us?
So shall my word be that goeth forth out of my mouth: it shall not return unto me void, but it shall accomplish that
which I please, and it shall prosper in the thing whereto I sent it. Isaiah 55:11
Blessed be the Lord, who daily loadeth us with benefits, even the God of our salvation. Selah. Psalms 68:19
But my God shall supply all your need according to his riches in glory by Christ Jesus. Philippians 4:19
Premise:
Food, respiration (re-spiriting) and water intake is not enough energy to maintain body temperature
let alone all other metabolic functions.

How much energy is required to maintain body temperature for a 150 lb man?
Oxidation of 1 g of dietary carbohydrate and 1 g of dietary protein each yield approximately 4 kcal (Calorie) and oxidation of 1 g of dietary fat yields approximately 9 kcal (Calorie).
Coroner Rule of Thumb = once life stops 1.5 °F per hour temperature loss
new specific heat of man = 2.98 kJ * kg-1 * °C-1
| 2.98 | kJ ---------- kg * °C |
* 150 | lbs | / 2.2 | kg --- lbs |
* 1.5 | °F ----- hour |
* 5/9 | °C --- °F |
* 0.239 | Cal ---- kJ |
= | 40.5 | Cal ----- hour |
= | 972 | Cal ---- day |
40.5 food Calories per hour to maintain body temperature loss of 1.5°F per hour. This presumes other metabolic or outside inputs need to maintain 25.1 °F over ambient (72 °F).
| 2.98 | kJ ---------- kg * °C |
* 150 | lbs | / 2.2 | kg --- lbs |
* 25.1 | °F ----- hour |
* 5/9 | °C --- °F |
* 0.239 | Cal ---- kJ |
= | 677 | Cal ----- hour |
= | 16,251 | Cal ---- day |
+677 food Calories to maintain body temperature of 97.1 °F, 25.1 °F over ambient (72 °F) per hour = 16,251 Calories per day.
|
https://pubmed.ncbi.nlm.nih.gov/37332308/
https://coronertalk.com/28 |
USA Army July 22, 2022 September 2018 |

Food, respiration (re-spiriting) and water intake is not enough energy to maintain body temperature let alone all other metabolic functions.
|
Standard Diet
-10% for digestion -daily to prevent 1.5 °F temperature drop per hour -daily to maintain temperature over 72 °F we need a lot more nothing left for all metabolic processes |
2,000 Calories
-200 Calories -980 Calories -16,250 Calories ============ -15,430 Calories |
Even if you add in that respiration is exothermic, there is a missing input.
The missing input obviously is not visible to our senses; but, yet it exists or we would not.
God's Love (life force) constantly supplies us with something to maintain our lives.
We as a living respiring man are inseparable from God's Love (life force).
This should make getting "saved" simpler.
If you are an atheist. ok, it comes from the universe; but, how does it know how to come at creation and leave at
death?

2.98 * 150 / 2.2 * (? * 5/9 ) * 0.239 = 27 Δ °F-1 Cal / hour
Summer: Air temperature 90 °F high, 70 °F low, ambient 80 °F
| 2.98 | kJ ---------- kg * °C |
* 150 | lbs | / 2.2 | kg --- lbs |
* 18.6 | °F ----- hour |
* 5/9 | °C --- °F |
* 0.239 | Cal ---- kJ |
= | 502 | Cal ----- hour |
= | 12,042 | Cal ---- day |
+502 food Calories to maintain body temperature of 98.6 °F, 18.6 °F over summer ambient (80 °F) per hour = 12,042 Calories per day.
Winter: Air temperature 50 °F high, 30 °F low, ambient 40 °F
| 2.98 | kJ ---------- kg * °C |
* 150 | lbs | / 2.2 | kg --- lbs |
* 58.6 | °F ----- hour |
* 5/9 | °C --- °F |
* 0.239 | Cal ---- kJ |
= | 1,581 | Cal ----- hour |
= | 37,942 | Cal ---- day |
+1,581 food Calories to maintain body temperature of 98.6 °F, 58.6 °F over winter ambient (40 °F) per hour = 37,942 Calories per day.
I recently saw the perfect example of how specific heat works that anyone who skates on a pond will tell you. If a leaf blows onto a frozen pond and sticks to the ice, on a sunny day the leaf will soak up the sun's energy and heat up to the point where a leaf can sink through an inch of ice in a few hours.

Food does not have enough energy to even keep us warm.
Where does the constantly required energy come from?
Is there only one source? Probably not.
Is it the Holy Spirit? No.
Man is comprised of body and soul.
Is it soul? If only warm blooded animals have a soul? Probably not.
Most folk believe that animals (warm and cold blooded) have a soul.
The presence of a soul is possibly related to; but, not the heat source.
Once you are dead, the soul leaves and the temperature goes to ambient.
When the soul is present, so is God's Love (life force) and the life force maintains a warm blooded temperature.
If the soul is not the source of the required energy it must be Etheric.
Are Spiritual and Etheric the same? Probably not.
Spiritual and Etheric does not exist in modern science.
Solar and cosmic radiation on a microscopic scale cause the DNA cell nucleus to vibrate in an electrically conductive salt solution encased in a cell wall membrane that is an electric insulator that has no where to go ergo it reduces to heat within the cell. Life is vibration. Dead cells by definition do not vibrate. The Secret of Life, Georges Lakhovsky

The physical man is defined by DNA as is the rest of the plant and animal world.
In DNA the atoms are fungible. I.e. If you need a carbon atom it does not matter if it came from a cheeseburger or a salad.
DNA, the definition of the physical man, is pure information without mass.
E = mc2 shows mass and time are related.
Information without mass DNA, the definition of the physical man, means man is outside of time, ergo eternal.

Transmutation is highly exothermic, probably to the extent of being fatal.
Cold fusion is transmutation without the heat.
Catalysts which improve reactions without being consumed can aid in this process.
Here however we are searching for large quantities of heat; but, it must be manageable at the cellular level.
Following the discovery that helium gas, previously treated as a separate element, evolved itself as one consequence of the disintegration of radium. Transmutation, till then laughed at as superstition of the alchemist, passed quietly into the region of accepted natural phenomena.
Food, water and air are our only sources for new atoms without transmutation.

Coroner Rule of Thumb = once life stops 1.5 °F per hour temperature loss
old specific heat of man = 3.47 kJ * kg-1 * °C-1
150 lbs man
| 3.47 | kJ ---------- kg * °C |
* 150 | lbs | / 2.2 | kg --- lbs |
* 1.5 | °F ----- hour |
* 5/9 | °C --- °F |
* 0.239 | Cal ---- kJ |
= | 47.1 | Cal ----- hour |
= | 1,130 | Cal ---- day |
47.1 food Calories per hour just to maintain body temperature loss of 1.5 °F per hour. This presumes other metabolic or outside inputs need to maintain 25.1 °F over ambient (72 °F).
| 3.47 | kJ ---------- kg * °C |
* 150 | lbs | / 2.2 | kg --- lbs |
* 25.1 | °F ----- hour |
* 5/9 | °C --- °F |
* 0.239 | Cal ---- kJ |
= | 788 | Cal ----- hour |
= | 18,924 | Cal ---- day |
+788 food Calories to maintain body temperature of 97.1 °F, 25.1 °F over ambient (72 °F) per hour = 18,924 Calories per day.
|
https://pubmed.ncbi.nlm.nih.gov/37332308/
https://coronertalk.com/28 |
USA Army July 22, 2022 September 2018 |

Large calorie (Cal) is the energy needed to increase 1 kg of water by 1 °C at a pressure of 1 atmosphere.
| 4.184 | kJ ---------- kg * °C |
* 150 | lbs | / 2.2 | kg --- lbs |
* 1.5 | °F ----- hour |
* 5/9 | °C --- °F |
* 0.239 | Cal ---- kJ |
= | 56.8 | Cal ----- hour |
= | 1,363 | Cal ---- day |
56.8 food calories per hour to maintain body temperature loss of 1.5 °F per hour. This presumes other metabolic or outside inputs need to maintain 25 °F over ambient (72 °F) = 1,363 Calories per day.
| 4.184 | kJ ---------- kg * °C |
* 150 | lbs | / 2.2 | kg --- lbs |
* 25.1 | °F ----- hour |
* 5/9 | °C --- °F |
* 0.239 | Cal ---- kJ |
= | 951 | Cal ----- hour |
= | 22,818 | Cal ---- day |
+951 food Calories to maintain body temperature of 97.1 °F, 25.1 °F over ambient (72 °F) per hour = 22,818 Calories per day.
Next I did it the old way, 1 calorie = 1 gram / °C.
| 1 | cal --- g |
* 150 | lbs | / 2.2 | kg --- lbs |
* 1.5 | °F ----- hour |
* 5/9 | °C --- °F |
* 1,000 | g --- kg |
/ 1,000 | Cal ---- cal |
= | 56.8 |
Wow, the same number. Then I did 4.184 * 0.239 = 1
It does not matter which conversion factor you use, they are all the same.

Heat Only
Maintaining 98.6 °F temperature requires 17,430 Calories for a 150 pound man with 70 trillion cells.
| 17,430 / 70,000,000,000,000 = 0.000000000249 | = 2.49 * 10-10 Calories per cell
= 2.49 * 10-13 calories per cell |
Is this manageable at the cellular level?

With 70 trillion cells duplicated over 7 years
| 70,000,000,000,000 / 7 years / 365 days/year | = .00273 x 1013 cells duplicated each day
= 2.73 x 1010 |
24 billion cells are duplicated each day.

The minimum energy needed to build a cell as defined here is the sum of the energy required to assemble all its components into their biomolecules.
At 298 °K, the energy required to synthesise one single E. coli cell is 9.54 x 10-11 J/cell (331 J/g)
and 3.69 x 10-12 J/cell (329 J/g) for JCVI-syn3A. For a mammalian cell, the energy required is 3.71 x 10-9
J/cell (354 J/g),
https://www.nature.com/articles/s41598-024-54303-6
To build a mammalian cell takes 3.71 10-7 J/cell at 298 °K
Is 2.49 * 10-13 calories per cell manageable at the cellular level?
| 1 kJ
1 J |
-> calorie
-> calorie |
0.2390057361
0.0002390057361 |
= 2.39 * 10-1
= 2.39 * 10-4 |
3.71 10-7 J * 2.39 * 10-4 calorie / J = 8.87 10-11 calories to create a mammalian cell.
If we need 400 times the amount of energy per cell to maintain temperature merely to create a single mammalian cell
let alone all other metabolic functions our net outside input must be substantially higher than previously calculated.
Physical activity creates heat. Athletes have warm ups.

Medical doctors use pharmacopia (Greek for witchcraft) and are so dangerous that they must be licensed. The AMA has admitted for years that iatrogenesis is the third leading cause of death. https://hub.jhu.edu/2016/05/03/medical-errors-third-leading-cause-of-death/ https://pubmed.ncbi.nlm.nih.gov/28186008/
Medical doctors claim separation of God from State prevents medical doctors from acknowledging God or any of God's effects on man.
Medical doctors are licensed. I.e. they are inferior with respect to their creator the licensor superior ergo they can't have more alleged rights than We the People surrendered to the government and simultaneously kept with the Ninth and Tenth Amendments. This has to be where the separation is.

Energy from the sun powers life on earth, and man is no exception.
Of course, everyone has seen ultraviolet rays create a suntan.
Doctors use Xrays, Cat scans, Pet scans and ultrasound waves to peer inside the human body without surgery.
A microwave heats by frequency energy converted directly to heat by absorption by molecules.
As man we see the result of what comes from the sun after it interacts with our atmosphere as visible light and feel the infrared heat waves.

Frequency energy within a certain frequency range of the electromagnetic radiation is what our eyes see as colors.
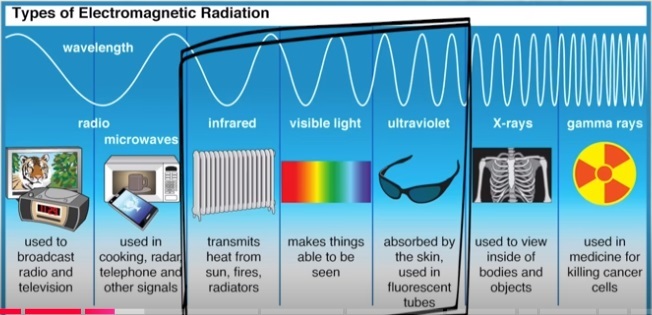
Before red is infrared and long waves with low energy.
After blue is short waves with high energy.
This varies as to which way is up depending whether you are looking at increasing frequency or increasing energy.
Light at the smallest level is a photon.
An atom is like a miniature solar system. However instead of planets there are electron levels. Each electron level can generally hold 8 electrons.
A photon is released when an electron within an atom with an outside energy input jumps to a higher electron level but does not have enough continued energy input to maintain the new electron level and falls back to the original electron level where it started releasing a photon in the process.
Fireworks utilize this process obtaining different colors with different molecular not physical metals. Physical metals behave very differently than molecular metals. The heat of the material burning creates the outside energy input to jump to a higher electron level.
A single photon is not visible, but lots of photons can be.
Light emitting diode (LED) phototherapy has been well-proven to have an effective benefit in a wide variety of clinical
indications such as pain relief, skin injuries, rheumatological diseases, muscle disorders, and infections, suggesting
that LED light might have a powerful role to play in the clinical practice for a variety of conditions.
https://onlinelibrary.wiley.com/doi/10.1155/2021/6663539
Sometimes the heart can see what the eyes can not. [Sign at a church]

Light is created at conception.
https://www.sciencealert.com/scientists-just-captured-the-actual-flash-of-light-that-sparks-when-sperm-meets-an-egg https://www.nih.gov/news-events/news-releases/zinc-sparks-fly-egg-within-minutes-fertilization
Mitochondria are often referred to as the “powerhouse of the cell” because they convert food, water, and oxygen into adenosine triphosphate (ATP) energy the cells and body can use.
Mitochondria are unique, with their own ribosomes and DNA.
ATP is often referred to as the "energy currency" of the cell. It powers various cellular processes, from muscle contractions to nerve impulses, making it essential for life. ATP is produced in the mitochondria, the cell's powerhouse, through a process known as cellular respiration. This complex process involves the conversion of glucose and oxygen into ATP, water, and carbon dioxide.
The sun is the most important source of energy for the earth and represents the engine of the climate as well as multiple biological processes.
Mitochondria can directly produce ATP from certain wavelengths of red and near-infrared light.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4387504/
https://www.geneticlifehacks.com/red-light-photobiomodulation-atp/
https://www.hotworx.net/blog/how-infrared-light-triggers-atp-production-unleashing-the-cellular-powerhouse
This is medical science showing red and near-infrared light possibly from outside of the body or created via interaction the of cosmic rays with DNA or from other biological processes are not only involved with life but critical thereto and previously ignored by science but shown to man since Old Testament times.
It is a bit mind-boggling that under the right conditions the mitochondria in our cells can generate energy from light. Is this is a part of our missing energy?
Infrared light is a part of the electromagnetic spectrum with wavelengths longer than visible light so infrared frequencies are invisible to our eyes.
Are invisible things like frequencies real? Ever listen to a radio or watch TV or use a cellphone or WiFi? Doctors use Xrays, Cat scans, Pet scans and ultrasound waves to peer inside the human body without surgery.
Slightly more than half of the solar radiation that passes through the atmosphere and reaches the Earth's surface is infrared ergo invisible.
Outside light can only penetrate the skin so far.
What if biological processes create light frequencies within cells which are used by adjoining cells which propagate like a nuclear reaction?
Infrared frequencies are heat but also activate further biological functions.
Infrared frequencies are generally invisible but in the summer heat waves can be seen coming off of highways where the heat affects the air above it.

Who would deny that prayer is an invisible link from us to God especially after Jesus rent the veil separating us from the Holy of Holies?
| I love to tell the story of unseen things above,
of Jesus and his glory, of Jesus and his love. I love to tell the story because I know it's true; it satisfies my longings as nothing else can do. |
| John 5:39 | Search the scriptures; for in them ye think ye have eternal life: and they are they which testify of me. |
| Ecclesiastes 12:6 | Or ever the silver cord be loosed, or the golden bowl be broken, or the pitcher be broken at the fountain, or the wheel broken at the cistern. |
| Ecclesiastes 12:7 | Then shall the dust return to the earth as it was: and the spirit shall return unto God who gave it. |
The Biblical silver cord is an invisible link from God to us.
If breaking the silver cord causes us to die the silver cord must be physical or metaphysical even if invisible. This can lead us into uncharted territory.
Unfortunately because of the invisibility of the Biblical silver cord it is often addressed only esoterically.
Lets take a skeptical look with an open mind at this science or esoteric wacky ideas to see if it can have some value to man today.
Old knowledge that was lost can be re-found. Comparing some esoteric wacky ideas to science may identify whether these ideas are correct or completely wrong.
Are invisible things like frequencies real?
Ever listen to a radio or watch TV or use a cellphone or WiFi?
If invisible frequencies delivered or accessed via the silver cord or otherwise link us to God and sustain us to the point where we die without it the silver cord is Biblical and must be real to our modern senses of frequencies even though invisible to our forefathers.
What we perceive through our senses as a hard wooden table is simply a set of fast moving very small whirls of energy bound by their common flows to other particles giving the appearance of solidity. This has been called the "Grand Illusion" among other things and reminds us that a real understanding of something in nature requires more than man's senses.
With cell phones all of the world can in real time see the anti-Christ walking into the Holy of Holies.
But when ye shall see the abomination of desolation, spoken of by Daniel the prophet, standing where it ought not, (let him that readeth understand,) then let them that be in Judaea flee to the mountains: Mark 13:14
In our age are we seeing things before unseen?
FLIR (forward looking infrared heat cameras) and night vision is know to most folk.
I have a sheet that when placed over a magnet allows you to see the invisible magnetic lines of force which is not magic but new technology using iron dust and oil encased in plastic.
Almighty God shows himself to us in myriad ways.
If I have told you earthly things, and ye believe not, how shall ye believe, if I tell you of heavenly things? John 3:12

Yes. God taught us about the spiritual world that is invisible to us.
Since the spiritual world is invisible to us it makes sense that there might be other things in the world invisible to us.
The biblical authors and those to whom they wrote were predisposed to supernaturalism. Just try to imagine being a part of some of the old and new testament stories and realize they really happened.
We have become desensitized to the vitality and theological importance of the unseen world.
Noncharismatics tend to close the door on the supernatural world because of their suspicion that charismatic practices are detached from a sound exegesis of Scripture.
Traditional Christian teachings for centuries kept the unseen world at arms length. We believe in the Godhead because there's no point to Christianity without it.
God has created a heavenly host of non-man divine beings whose domain is (to human eyes) an unseen realm.

Stem cells are unique as no other cell in the body has the natural ability to generate new cell types.
The body can produce its own stem cells in the quantity it needs and direct them to wherever they’re required, as long as a sufficient number of silver ions are present in the bloodstream.
It has been proven that mans body can predict exactly the number of stem cells needed to heal a wound. Under the right conditions, the body can produce exactly that number, use them all and have no extra left over!
Still more surprising is that you can easily do the same thing yourself at home.
Dr Becker showed that in the presence of sufficient silver ions the body would produce all the stem cells it needed.
Copper was said to produce an uncontrollable decay of the flesh, and a wound from a copper sword was dreaded. Supposedly the only way to kill the fallen angels and demons is with pure copper bullets.
The Use of Colloids in Health and Disease (1919) states: "Applying colloidal silver to human subjects has been done in a large number of cases with astonishingly successful results." https://web.archive.org/web/20151030194125/http://www.blog.missionsnet.org/?p=326

Here is where some might consider we are getting esoteric or is our science learning about realms previously invisible to our physical senses?
Remember to never limit God to our understanding. John 3:12
Unfortunately those in Christ Jesus service that try to learn about God can mainly find information relating to the Biblical silver cord from sources most of those in Christ Jesus service would find questionable.
“Or ever the silver cord be loosed…Then shall the dust return to the earth as it was: and the spirit shall return unto God who gave it." Ecclesiastes 12:6,7
The main role of the silver cord is to nourish the subtle and physical bodies with vital energetic life force. Its function is to keep your incarnated soul attached and grounded to your physical body through the infusion of the Universal Life Force.
The Silver Cord is an integrated, energetic and fluidic threaded structure of energy filaments that connect the physical and ethereal bodies to the higher self during a man’s lifetime of incarnation. https://www.themysticmedium.com/the-silver-cord/
The silver cord is often referred to as the “life thread” because it supplies energy to the physical body. If the silver cord is severed, the physical body can no longer be sustained and dies.
Did your biology teacher or medical doctor or pastor ever tell you this?
Now science tells us that this previously thought weird idea might actually be true and Biblical as well.

The sole purpose of the silver cord, or life thread, is to provide the subtle and physical bodies with vital energy. Prana is a Sanskrit term meaning “life.” Without Prana, or spirit, the physical body could not operate as it does. This is why once the silver cord is severed, the physical body has no choice but to die.
Think of the physical body as a sensitive electromagnetic vehicle which filters and grounds spiritual energy and consciousness. By filter I mean that higher (subtle) energy is stepped-down (by chakras) through each subtle body until it manifests in the physical body. Denser matter is the most restrictive, so energy and consciousness is more limited in the physical body than in our subtle bodies. Although the physical body is more restrictive, it provides a highly varied experience for consciousness, so more restriction isn’t necessarily a bad thing.

Photobiomodulation is a form of light therapy that utilizes non-ionizing forms of light sources including lasers, LEDs, and broadband light, in the visible and near infrared spectrum. Ionizing radiation destroys cells.
Different wavelengths have different effects.
| Blue end | short wave length and higher energy |
| Red end | long wave length and lower energy |
Blue (415 nm) and green (540 nm) wavelengths were more effective in stimulating osteoblast differentiation of human adipose-derived stem cells (hASC) cultured in osteogenic medium, compared to red (660 nm) and near-infrared (NIR, 810 nm) light. Intracellular calcium was higher after 415 nm and 540 nm.
4 different wavelengths of light (3 J/cm) to stimulate the cells at 5 time points (48 h, 24 h, 6 h, 3 h,1 h) before the SRB assay. 3 J/cm of 810 nm and 660 nm laser promoted hASCs proliferation, but 415 nm and 540 nm wavelengths showed an inhibitory effect on proliferation at the same dose.
3 J/cm of 660 nm and 810 nm PBM could increase intracellular ATP level by 15–20%, while 3 J/cm of 415 nm and 540 nm
decreased intracellular ATP level in the region of 10%, 3 hours after irradiation.
https://pmc.ncbi.nlm.nih.gov/articles/PMC5552860/
455-nm blue light is effective for promoting proliferation, migration, and tube formation of HUVECs co-cultured with
Mesenchymal stem cells.
https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1472-x
Complex IV: “acts as a photo acceptor at 632.8 nm due to two heme A moieties and two copper centers.” The research s
hows that complex IV absorbs light at 620 – 680, and 760 – 895 nm (red and infrared). In addition, cytochrome b in
complex III is activated at 980 nm (infrared). To add a little more complexity to this, light in the 400–500 nm
wavelengths excites flavins, such as FAD from riboflavin.
https://pubmed.ncbi.nlm.nih.gov/16144476/
660 nm has multiple studies.
https://www.jkslms.or.kr/journal/view.html?doi=10.25289/ML.2020.9.2.134
Stimulation of cellular functions is seen at specific wavelengths: between 613 and 623 nm, between 750-772 nm, and
between 812-846 nm.
https://pubmed.ncbi.nlm.nih.gov/16144476/
808 and 810 nm confirmed that the application of photobiomodulation formerly known as low-level laser therapy (LLLT)
with the red wavelength is very effective for the differentiation and proliferation of stem cells.
https://www.jkslms.or.kr/journal/view.html?doi=10.25289/ML.2020.9.2.134
810 nm had a peak dose response at 3J/cm2 for stimulation of proliferation at 24 hours.
https://pmc.ncbi.nlm.nih.gov/articles/PMC5195895/
Bone marrow stem cells are able to support angiogenesis and enhance the formation of new microvessels, secrete nerve
growth factors and restore nerve function after ischemic stroke, as well as used to treat non-healing fractures.
Significant results came from the use of wavelengths in the near infrared region (808, 890, 905 and 1064 nm), which
showed remarkable results in wound healing and bone formation.
https://www.jkslms.or.kr/journal/view.html?doi=10.25289/ML.2020.9.2.134
The 980 nm light produced a cellular response (caused the stem cells to differentiate) that was dependent on the
activation of calcium ion channels and temperature. A calcium channel blocker could block the effect of the 980nm
light, and cold or heat could also block the effect.
https://pubmed.ncbi.nlm.nih.gov/27751953/
980 nm (but not 810 nm) increased cytosolic calcium while decreasing mitochondrial calcium. The effects of 980 nm could be blocked by calcium channel blockers.
The peak dose for 980 nm was 10–100 times lower at 0.03 or 0.3 J/cm2.
https://pmc.ncbi.nlm.nih.gov/articles/PMC5195895/
Stem cell therapy needs a lot of human cells, of a specific type. Shining a near-infrared laser on adult stem cells derived from human body fat, makes the stem cells replicate 54% faster. Following that up with a green laser, enables the stem cells to transform into different kinds of cells faster and more reliably.

Photodynamic therapy is a technique by which certain chemical agents, known to perfuse well into tumors, are activated by laser light to generate oxygen free radicals which in turn damage DNA and induce apoptosis (programmed cell death) of the tumor cells. This procedure requires the insertion of a fiber optic laser probe into tissue, and is only effective against early stage and localized disease.
Sonodynamic therapy (focused ultrasound with dye) may be able to activate sonosensitizers to induce cell death in tumors.
Sonodynamic therapy could offer advantages as compared to photodynamic therapy by activating these chemical agents in a noninvasive manner. Focused ultrasound has the capability of treating regions deeper in the body where light would either be blocked or require more invasive delivery methods.
https//www.fusfoundation.org/the-technology/mechanisms-of-action/sonodynamic-therapy/

Not all frequencies are beneficial.
Some frequencies are Harmful to Man. (Havana syndrome, LRAD, 5G)
This brings up psychotronics and MKULTRA.

Generally, researchers shine red and near-infrared laser light on stem cells in the laboratory. These wavelengths make the cells multiply into more identical stem cells. The process is called proliferation.
However, to repair any part of the body, it is also necessary to change stem cells into the other kinds of cells needed. That process is called differentiation.
But red and near-infrared laser light don't seem to encourage differentiation so much.
Green laser light (525 nanometer wavelength) on adipose-derived stem cells and near-infrared (825 nanometer
wavelength) laser light shown consecutively on stem cells make adipose-derived stem cells multiply rapidly. It also
encourages sufficient differentiation.
https://www.news-medical.net/news/20211026/Consecutive-irradiation-with-two-lasers-helps-adult-stem-cells-to-transform-faster.aspx

Bioluminescence is produced in living organisms such as fireflies and this should not be confused with biophotons.
In 1926, Russian embryologist Alexander Gurwitsh discovered the emission of light from cells in a living organism (Gurwitsch, 1934). He called them mitogenic.
Then in 1974 German researcher and Nobel Prize nominee in Physics Fritz Albert Popp re-confirmed the existence of these mitogenic rays and renamed them biophotons. He verified that all biological systems transmit light and information and that these subatomic light particles emanate from every living system, including from the cells and DNA of our bodies.
He identified the vibrating behavior of formerly called Junk DNA as the primary source of ultra-weak light emission, also called biophotons.
Biophotons can now be detected radiating from our hearts and brains.
Whoa - just a second. Your brain emits light? Really? And your heart does too? WOW. https://www.linkedin.com/pulse/biophotons-how-you-can-positively-influence-others-world-comaford/
Biophotons are photons (light particles) that are generated within the body, and these could be measured as they emanate from the skin.
Any stress to the skin in the form of exposure to ultraviolet radiation or cigarette smoke enhances the emission of biophotons while topical application of ascorbic acid or antioxidant solutions reduces such radiation.
Biophotons are light emissions that are extremely weak which means that biophotons cannot be observed by the naked eye.
Since biophotons are a result of oxidative processes also, there could be complex interrelation between oxidative
processes, biophotons, and Qi energy.
https://web.archive.org/web/20241214115749/https://pmc.ncbi.nlm.nih.gov/articles/PMC5433113/
Mothersill and many others during the last hundred years have shown that cells and now whole animals may communicate with each other by electromagnetic waves called biophotons. This would explain the source of the bystander phenomena. These ultra-weak photons are coherent, appear to originate and concentrate in DNA of the cell nucleus and rapidly carry large amounts of data to each cell and to the trillions of other cells in the human body. The implications of such a possibility can be wonderfully important. https://web.archive.org/web/20241227105306/https://pmc.ncbi.nlm.nih.gov/articles/PMC4267444/
Cells emit light at ultra-low intensities: photons which are produced as by-products of cellular metabolism, distinct from other light emission processes such as delayed luminescence, bioluminescence, and chemiluminescence. The phenomenon is known by a large range of names, including, but not limited to, biophotons, biological autoluminescence, metabolic photon emission and ultraweak photon emission (UPE), the latter of which shall be used for the purposes of this review. It is worth noting that the photons when produced are neither 'weak' nor specifically biological in characteristics. https://web.archive.org/web/20241211030559/https://pmc.ncbi.nlm.nih.gov/articles/PMC10899412/
"We know today that man, essentially, is a being of light. And the modern science of photobiology … is presently proving this. In terms of healing, the implications are immense. We now know, for example, that quanta of light can initiate, or arrest, cascade-like reactions in the cells, and that genetic cellular damage can be virtually repaired, within hours, by faint beams of light. We are still on the threshold of fully understanding the complex relationship between light and life, but we can now say emphatically, that the function of our entire metabolism in dependent on light." Dr. Fritz-Albert Popp

Extremely low frequency electromagnetic fields promote mesenchymal stem cell migration by increasing intracellular Ca2+ and activating the FAK/Rho GTPases signaling pathways in vitro. https://stemcellres.biomedcentral.com/articles/10.1186/s13287-018-0883-4

Man is an antenna. Some folk can remember rabbit ears and AM radios where touching the TV or radio makes the reception better by extending the antenna to include you.

The concept of absolute zero means that anything above absolute zero emits energy.
Can some men see colors coming off of other men that most men can not see? Many say yes.
Though aura as a subject is seems to stand in opposition to modern day science, scientists now are beginning to believe in energies.
Most folk that see auras expect auras and if you ask where they come from they will say, you. How? We don't worry about that, we just read them.

Science is the study of the mechanics of God.
To the extent that the science is true, science accurately observes the structure of God's Creation.
In 1994 a team of researchers at the National Institute of Health identified the "biofield".
Infrared light radiation from the sun creating ATP within man's cells makes the sun a nutrient and a part of my missing energy.
I have no doubt that much of what I've presented is at best incomplete, and at worst woefully wrong, even though no one has identified it as wrong yet. That's ok. Since no one is remotely looking at this I'm much more afraid of saying nothing than I am of saying something incorrect.
If my work is correct, it requires reintegration of God in medicine at a minimum and actually subservience of medicine to God.
My hope is that minds are opened to new possibilities with God.